
Examining Ricin and Its Vaccine Derivatives, RiVax and RTA1-33/44-198
Ricin is a toxin that comes from castor beans and acts by inhibiting protein synthesis. It does so at the ribosomal level, which classifies it as a Type-2 Ribosomal-inactivating protein (RIP). It is differentiated from Type-1 RIPs by the presence of both an A chain and a B chain. In ricin’s case, the A chain acts as the toxic chain; however, what makes it so deadly is actually the A-B combination, since the B chain acts as a way of transporting the A-B protein complex from the cell surface to the lumen of the endoplasmic reticulum (Wright & Robertus 1987). On its own, the A chain is not actually toxic, and can in fact be found in other substances such as wheat and barley (Cornell University 2014). The ricin toxic A-chain (also known as RTA) is comprised mostly of alpha helices and beta sheets, while the B chain is less structured and contains only a few small alpha helices. This overall form can be seen in Figure 1. The isoforms that will be explored in this paper are not naturally occurring, but rather are man-made forms that are being used as vaccines against ricin poisoning. These vaccines are made from only the A chain of ricin, with either mutations at specific residues or modifications of chain length. These isoforms are known as RiVax and RTA1-33/44-198.
Figure 1: Ricin with B chain on left and A chain on right. PDB: 2AAI
An interesting derivative of Ricin, RiVax is structurally almost identical to ricin’s A chain. At residue 76, a valine has been substituted for a methionine and at residue 80, a tyrosine has been substituted for an alanine (Legler et al., 2011). These two substitutions have been made to reduce the toxicity; the Y80A substitution reduces the catalytic activity of ricin that is required for it to act as an RIP, while the V76M substitution helps to protect against activity that could cause vascular leak syndrome in humans (Janosi et al., 2013). The two mutated residues have been highlighted below in Figure 6 to demonstrate the differences between ricin and its potential vaccine.
In addition to RiVax, however, RVEc is also being developed as a potential vaccine against ricin. Currently, it has been shown to protect non-human primates from ricin but is still being assessed as a possible vaccine for humans (Janosi et al., 2013). In contrast to ricin and RiVax, however, it includes only residues 1-33 and 44-198 of the normal ricin A chain, making it considerably smaller than either of them. In addition to this difference, what also makes it unique from the other two forms is the disulfide linkage added between R48C/T77C, which is shown in Figure 7 (Janosi et al., 2013). The numerous deletions as well as added linkage have been engineered in order to help stabilize RVEc and potentially make it a morecost-efficient vaccine than RiVax. Janosi et al. have also experimented with different forms of RTA1-33/44-198 to test stability and compatibility with the introduced disulfide linkage. One site of experimentation is shown in Figure 8.

RiVax, the first of the two isoforms, is still under clinical development. Compared to the other two isoforms, RiVax is smaller than ricin but larger than RTA1-33/44-198. Structurally, it is composed only of the A chain of ricin, but with two substitutions made aimed at reducing toxicity. The overall structure of Ricin can be seen in Figure 2. The other isoform, RTA1-33/44-198 (also known as RVEc), is the smallest of the three isoforms and varies to a much greater degree than the others. It is made of a much shorter version of the A chain, and incorporates a new disulfide linkage to stabilize the molecule. This stabilization also helps to increase solubility, which would help with its effectiveness as a vaccine. The overall structure includes fewer amino acid chains, resulting in a structure that is primarily alpha helices and beta sheets without other chains that lack secondary structure.

RiVax, the first of the two isoforms, is still under clinical development. Compared to the other two isoforms, RiVax is smaller than ricin but larger than RTA1-33/44-198. Structurally, it is composed only of the A chain of ricin, but with two substitutions made aimed at reducing toxicity. The overall structure of Ricin can be seen in Figure 2. The other isoform, RTA1-33/44-198 (also known as RVEc), is the smallest of the three isoforms and varies to a much greater degree than the others. It is made of a much shorter version of the A chain, and incorporates a new disulfide linkage to stabilize the molecule. This stabilization also helps to increase solubility, which would help with its effectiveness as a vaccine. The overall structure includes fewer amino acid chains, resulting in a structure that is primarily alpha helices and beta sheets without other chains that lack secondary structure.
Figure 2: RiVax with mutations to reduce toxicity; similar in size to RTA. PDB: 3SRP.

Ricin is a unique protein in that it is actually in the midst of being researched. It is particularly interesting since it is incredibly deadly while also not being something that can be spread by contact, but rather used in more targeted attacks (such as against persons of interest or military personnel). This makes a potential vaccine against ricin something that – unlike flu or other more common vaccines – would be used in more limited capacities, for a much smaller target group. Historically speaking, it has been used in a few successful assassinations, and most recently an attempted one on the president (NBC News 2013). Ricin’s toxicity comes from its depurination of adenosine in ribosomal RNA at the key sequence known as the sarcin-ricin loop. This depurination can be done up to 1500 of times per minute. (Lord et al., 2003; Sperti et al., 1973). John H. Carra and colleagues explored various interactions ricin may have with amino acids in an effort to determine the minimal set of bonding interactions required to induce rearrangements on the ribosome (Carra et al, 2007). Ricin’s interactions with adenine can be seen in Figure 4 and a primary adenine target in ribosome is highlighted in Figure 5. In addition to its activity as an RIP, ricin also has a residual motif that can potentially cause vascular leakage in humans, if the ricin has not already killed its host (Janosi et al., 2013).
Figure 3: RTA1-33/44-198 with shorter chain and new disulfide linkage. PDB: 4IMV.

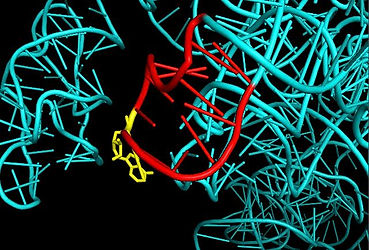
Figure 4: Ricin interaction with adenine residue (Carra et al., 2007). PDB: 2P8N.
Figure 5: The Sarcin- Ricin Loop in ribosomes is shown in red, while the A-3027 residue which is depurinated is shown in yellow. PDB: 3U5D.

Figure 6: On the left, Ricin (PDB:2AAI) with V76 and Y80; on the right RiVax (PDB: 3SRP) with V76M and Y80A substitutions.


While both RiVax and RVEc appear to be potential vaccines against ricin, they both have potential downsides. RiVax is known to be effective in humans, but still requires a large number of residues, which makes it a more costly vaccine to produce (see: Figure 9); RVEc is more cost-efficient, but may still cause vascular leaking if given in large enough doses. What Janosi et al. hope to find through research is a way to combine methods used to create both vaccines in order to produce a cost-efficient, completely safe vaccine for humans.
In addition to ricin derivatives being used against ricin itself, there is also notable research currently being conducted that would alter ricin in a way that turns it into a sort of anti-cancer “magic bullet” (Actionbioscience 2014). While no official research has been published, Dr. Ellen Vitetta has discussed the possibility of using ricin in conjunction with tumor-seeking antibodies. This would allow the antibody portion of the complex to seek the cancer cells while the RTA portion would deliver the toxin directly into the cells, killing them, but also having no adverse effects on surrounding proteins. An artist’s rendition of this sort of immunotoxin complex can be seen in Figure 10.
Figure 8: V76 in RVEc. In other forms of the RTA1-33/44-198 protein, this has been substituted with an isoleucine but compatibility with the disulfide bond was reduced in the other form. PDB: 4IMV.
Figure 7: R48C/T77C disulfide linkage in RVEc to stabilize the protein. PDB: 4IMV.


Figure 10: Rendition of ricin as an immunotoxin. The antibody (yellow) is modified with the ricin A chain (red) as a way to directly target cancer cells, bind to them, and then deliver ricin’s toxin to eliminate them (Goodsell, D.S. 2013).
Figure 9: RiVax residues 34-43 highlighted; Valine-Arginine and Isoleucine-Proline residues constitute small Beta sheets (green) that are not present in RTA. These additions are part of what make RiVax less cost-efficient than RVEc.
In doing this research, I have learned much more about the specific mechanisms by which ricin works; how it binds, what it disrupts, and why this is so deadly. This knowledge has helped me to also better understand how and why vaccines are being developed to combat its deadly effects. Prior to this research, I was not aware vaccines were being made against ricin, nor was I aware of what kind of research took place when creating vaccines of this nature.